Here are the calculations that I used in my presentation at ASA 2025.
pH versus PaCO2
An increase of 10 mmHg in PaCO2 results in a pH drop of about 0.08
Respiratory Acidosis PaCO2 vs HCO3–
In Acute Respiratory Acidosis (e.g. patient hypoventilating in the OR) the Bicarbonate Increases by 1mmol/L for every 10mmHg Increase in PaCO2
In Chronic Hypercarbia (COPD) the Bicarbonate ↑ by 4mmol/L for every 10mmHg Increase in PaCO2 and Cl– falls by an equivalent amount
Modern Anion Gap = [Na+ + K+] – [Cl– + HCO3– + La–+ βOH–] = Albumin– + PO42- + UMA– mEq/L
[Albumin−]= [Albumin g/L] × (0.123 × pH−0.631)
Albumin Charge is simplified to 2.5 (albumin in g/dl) = Alb in mEq/L
Change in Albumin is (44 – Albumin) / 4
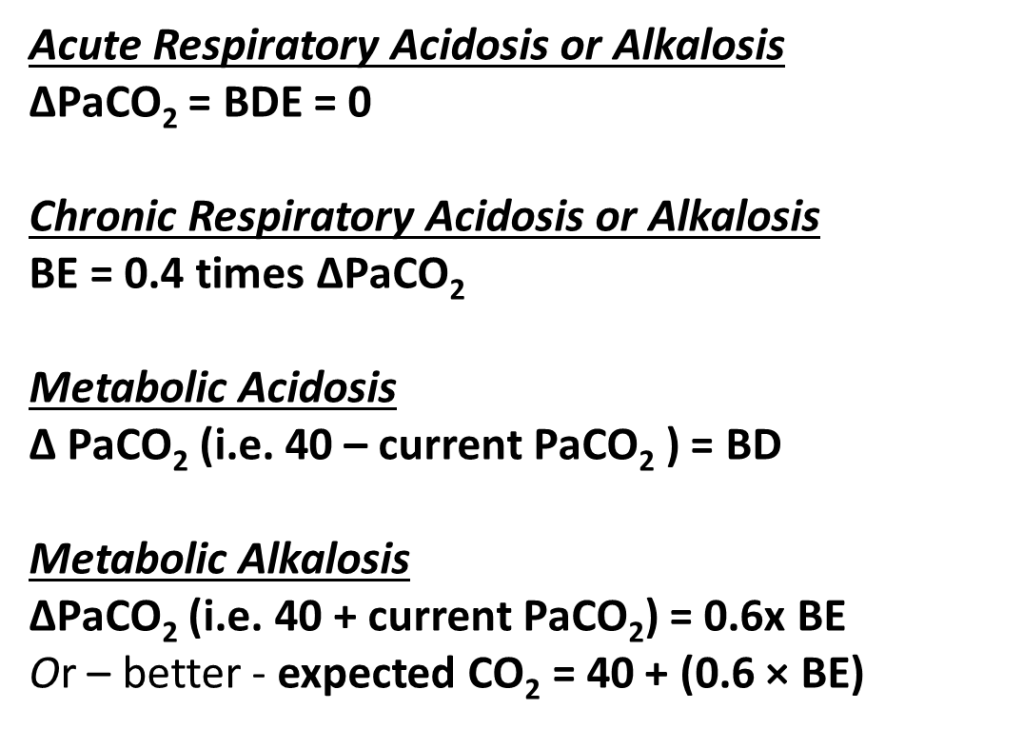
To determine the effectiveness of respiratory compensation use the Winters formula:
(Bicarbonate Version) Expected PaCO2 in Acute Metabolic Acidosis is
1.5 x [HCO3–] + 8 (in mmHg)
(Base Deficit Version) Expected PaCO2 in Acute Metabolic Acidosis is
Normal PaCO2 – BD (in mmHg)
The Base Excess Gap (Fencl Story with my modification)
Identify the BE on the ABG
Calculate the SID for Na+-Cl–+H2O by [Na+ – Cl– – 35] BDNaCl
Calculate the SID for La– and β-OH– (1mmol = 1mEq) BDLβOH
Calculate the Impact of Albumin (44 – Alb– g/L)/4 BEALB
Add these together BENaCl BDLβOH BEALB
Subtract from BE on the Blood Gas
The result is UMA in mEq/L
Finally, the Strong Ion Gap (arguably the gold standard)
The calculation for the strong ion gap (SIG) is:
Strong Ion Gap (SIG) = SIDa-SIDe
SIDa (apparent SID) = ([Na+] + [K+] + [Mg2+] + [Ca2+]) – ([Cl–] + [Lactate–] + [βOH–])
SIDe (effective SID) = [HCO3–] + [charge on albumin] + [charge on Pi]
The degree of ionization for weak acids is pH dependent, so one must calculate for this:
[charge on albumin] = [albumin] (in g/L) x (0.123 x pH – 0.631)
[charge on Pi] = [Pi] (in mg/dL) /10 x pH – 0.47
The SIG quantifies UMA
